
research
Vacuum Ultraviolet Absorption Spectroscopy
We are working at the interface of chemistry and physics, carrying out vacuum ultraviolet spectroscopic investigations of electronic structure for a variety of simple molecular fluids. Studies focus on temperature and pressure dependence of absorption spectra up to and exceeding the thermodynamic critical point. Density-dependent studies above the critical temperature give direct insight into the nature of intermolecular forces. Results are compared to computational models of electronic structure and intermolecular potentials.

Perturbation of Supercritical Carbon Dioxide Absorption Spectra
For supercritical carbon dioxide at 308 K, a gradual increase in pressure quenches the vibrational structure apparent in the gas phase due to nearest neighbor interactions
Pressurized and Supercritical Carbon Dioxide
Carbon dioxide is prevalent in planetary atmospheres and sees use in a variety of industrial applications. Despite its ubiquitous nature, its photochemistry remains poorly understood.
We explored the density dependence of pressurized and supercritical carbon dioxide electronic absorption spectra by vacuum ultraviolet spectroscopy over the wavelength range 1455-2000 Å. We showed that the lowest absorption band transition energy is unaffected by a density increase up to and beyond the thermodynamic critical point (137 bar, 308 K). However, the diffuse vibrational structure inherent to the spectrum gradually decreases in magnitude. This effect cannot be explained solely by collisional broadening and/or dimerization. At high densities, close proximity of neighboring molecules with a variety of orientations perturbs the multiple monomer electronic state potential energy surfaces, facilitating coupling between binding and dissociative states. A critical intermolecular radius of ~4.1 Å is necessary to cause such perturbations.
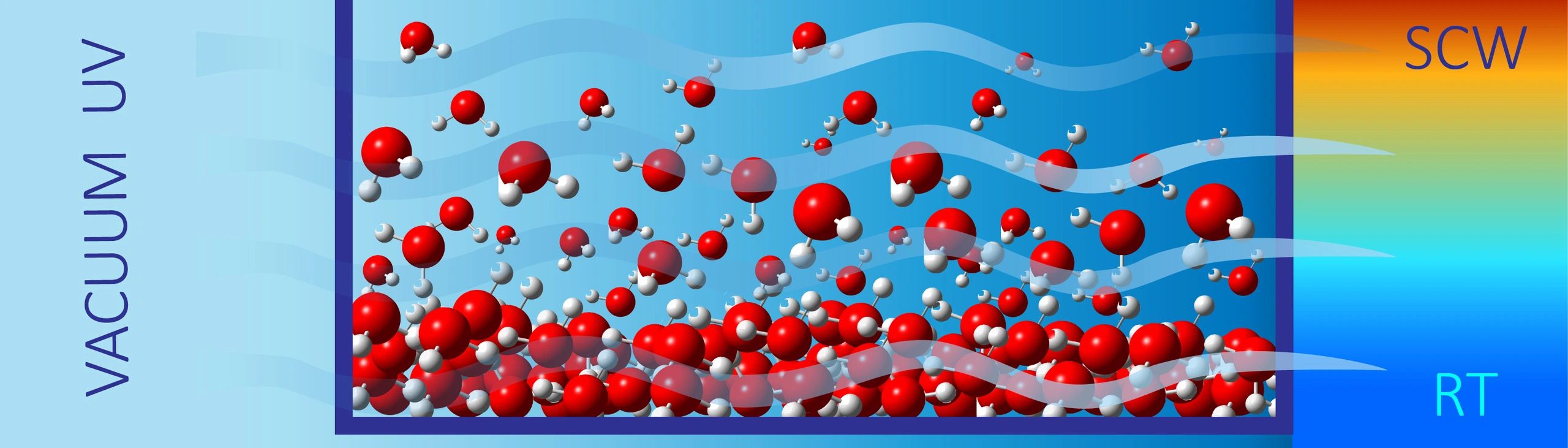
Subcritical and Supercritical Water
The nature and extent of hydrogen bonding in water has been scrutinized for decades, including how it manifests in optical properties.
We reported vacuum ultraviolet absorption spectra for the lowest-lying electronic state of subcritical and supercritical water (SCW). For subcritical water, the spectrum redshifts considerably with increasing temperature, demonstrating gradual breakdown of the hydrogen-bond network. Tuning the density at 381 °C gives insight into the extent of hydrogen bonding in SCW. The known gas-phase spectrum, including its vibronic structure, is duplicated in the low-density limit. With increasing density, the spectrum blueshifts and the vibronic structure is quenched as the water monomer becomes electronically perturbed. Fits to the SCW spectra demonstrate consistency with dimer/trimer fractions calculated from the water virial equation of state and equilibrium constants. Using the known water dimer interaction potential, we estimate the critical distance between molecules (ca. 4.5 Å) needed to explain the vibronic structure quenching.

Perturbation of Supercritical Water Absorption Spectra
For supercritical water at 653 K, with increasing pressure above the critical temperature, spectra broaden and blueshift due to dimer and trimer formation.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.